Special report: "The Séralini affair
"Some of the most important publications by scientific teams supported by CRIIGEN or by CRIIGEN members who have participated in them.
Traditionally, when a scientific study is cited, only the name of the first author is given. Exceptionally, all authors are cited to thank them for their efforts, cooperation and support of CRIIGEN.
(Authors who are members of CRIIGEN are underlined)
Since 2005, we have understood that the toxicological standards for pesticides and GMOs are inadequate to protect the world's population. A pesticide is composed of a molecule declared active plus co-formulants considered neutral...
Official toxicological standards for toxicity testing require only one test on rats for 2 years, and only on the molecule declared active by the manufacturer.
For example: Roundup and glyphosate-based herbicides, the best-selling herbicides in the world, are composed of glyphosate + co-formulants, which vary from one Roundup to another. Since 2005, we have shown that the toxicity of Roundups is greater than that of glyphosate alone.
In fact, pesticides were and still are tested only for 2 years on the molecule declared active.
Whereas GMOs, glyphosate-tolerant plants, were and still are only tested for three months on rats.
Based on these findings, in 2007 we decided to carry out a " world first ", i .e. to test a GMO and its associated pesticide on rats for two years. Since 2007, this experiment has never been repeated, and we are now in 2023...
Publication of this study in 2012 in "Food and Chemical Toxicology:
Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Gilles-Eric Séralini, Emilie Clair, Robin Mesnage, Steeve Gress, Nicolas Defarge, Manuela Malatesta, Didier Hennequin, Joël Spiroux de Vendômois. (2012). Food Chem. Tox. 50, 4221-4231.
This is the first study in the world to test Roundup and the glyphosate-tolerant GMO for two years...
A year after its publication, and after numerous attacks on the study, the director of Food and Chemical Toxicology withdrew the publication on the grounds that it was not conclusive, stating that there had been no miscalculation or malpractice...
Monsanto and the global agrochemical industry have been at work to make this publication disappear...
Details in: "L'Affaire Roundup à la lumière des Monsanto Papers" G-E Séralini Acte Sud 2020.
Exceptionally, our publication was republished in 2014 under the title:
Conflicts of interests, confidentiality and censorship in health risk assessment: the example of an herbicide and a GMO. Gilles-Eric Séralini, Robin Mesnage, Nicolas Defarge, Joël Spiroux de Vendômois. Editorial Environ Sci Eur. 2014. doi: 10.1186/s12302-014-0013-6.
" Conflicts of interest, confidentiality and censorship in health risk assessment: the example of a herbicide and a GMO ".
The health effects of Roundup-tolerant genetically modified (GM) NK603 maize (from 11% in the diet), grown with or without application of Roundup and Roundup alone (from 0.1 ppb of the complete pesticide containing glyphosate and adjuvants) in drink and water, were evaluated for 2 years in rats...
... This study is a follow-up investigation to a 90-day feeding study conducted by Monsanto to obtain commercialization of this GMO, using the same strain of rat and analyzing biochemical parameters on the same number of animals per group as our investigation. Our research represents the first chronic study of these substances, in which all observations, including tumors, are reported chronologically. It was therefore not designed as a carcinogenicity study. We report the main findings, with 34 organs observed and 56 parameters analyzed at 11 time points for most organs.
Results: Biochemical analyses confirmed very significant chronic renal insufficiency, for all treatments and for both sexes; 76% of altered parameters were related to the kidneys. In treated males, hepatic congestion and necrosis were 2.5 to 5.5 times higher. Marked and severe nephropathy was also generally 1.3- to 2.3-fold higher. In women, all treatment groups showed a two- to three-fold increase in mortality, with deaths occurring earlier. This difference was also evident in three groups of men fed GM corn. All results were hormonal and sex-dependent, and pathological profiles were comparable. Females developed large mammary tumors more frequently and before controls; the pituitary gland was the second most impaired organ; sexual hormonal balance was altered by GM corn consumption and Roundup treatments. The men had palpable tumors up to four times larger, starting 600 days earlier than in the control group, in which only one tumor was noted. These results may be explained not only by the non-linear endocrine-disrupting effects of Roundup, but also by overexpression of the EPSPS transgene or other mutational effects in GM maize and their metabolic consequences.
Conclusion: Our results imply that long-term feeding trials (2 years) should be carried out to thoroughly evaluate the safety of genetically modified foods and pesticides in their complete commercial formulations..
Between 2012 and 2014, we published numerous scientific articles in peer-reviewed journals...
2013
Answers to critics: Why there is a long term toxicity due to a Roundup-tolerant genetically modified maize and to a Roundup herbicide. Gilles-Eric Séralini, Emilie Clair, Robin Mesnage, Steeve Gress, Nicolas Defarge, Manuela Malatesta, Didier Hennequin, Joël Spiroux de Vendômois (2013). Food Chem. Toxicol. 53,461-468.
" Answers to critics: Why there is long-term toxicity from genetically modified Roundup-tolerant corn and Roundup herbicide "
Our recent work (Séralini et al., 2012) remains the most detailed study to date of the lifelong consumption of a genetically modified agricultural organism (GMO) and its associated herbicide. Nevertheless, this study has generated a violent polemic against us. We have responded to all the attacks in this publication...
This is particularly true for NK603 maize, for which only one 90-day marketing test has previously been carried out with the same rat strain (Hammond et al., 2004). This is also the first detailed long-term research on mammals exposed to a highly diluted pesticide in its total formulation with adjuvants. This may explain why 75% of our initial criticisms within a week, among the publication's authors, came from plant biologists, some developing patents on GMOs, even though this was a toxicological article on mammals, and the Monsanto company, which owns both NK603 GM maize and Roundup (R) herbicide. Our study has limitations like any other, and here we carefully respond to all the criticisms from agencies, consultants and scientists that have been directed at the publisher or ourselves. At this level, a full debate would be biased if the mammalian toxicity tests of NK603 and R obtained by Monsanto were to remain confidential and therefore unavailable in electronic format for the entire scientific community to conduct an independent review of the raw data. In our article, the conclusions on the long-term toxicity of NK603 and Roundup were based on statistically highly discriminating results at biochemical level in the treated groups compared to controls, as these results corresponded well in blind analysis to the pathologies observed in the organs, i.e. were in turn linked to deaths by anatomopathologists. GM NK603 and GM R cannot be considered safe at this time.
2014
Letter to the Editor regarding " Delaney et al., 2014 ": uncontrolled GMOs and their associated pesticides make the conclusions unreliable. Robin Mesnage, Nicolas Defarge, Joël Spiroux de Vendômois, Gilles-Eric Séralini. Food Chem Toxicol 2014. Doi : 10.1016/j.fct.2014.07.003
" Letter to the editor regarding "Delaney et al., 2014": uncontrolled GMOs and their associated pesticides make conclusions unreliable ".
We are concerned about the characterization of the diet tested in the study by Delaney et al. (2014), investigating the subchronic health effects of genetically modified Roundup-tolerant canola (DP-Ø73496-4) on rats. The conclusion can be used by regulatory authorities. Purina Certified Rodent LabDiet 5002 was not tested for the presence of other Roundup-tolerant GMOs and Roundup herbicide residues. According to our accredited PCR analyses, this control diet also contained 18% NK603 Roundup-tolerant maize and 14.9% MON810 (a GMO-producing modified Bt insecticide). We also found 110 ppb glyphosate and 200 ppb AMPA (the main metabolite of glyphosate). Although their toxicities are debated (Seralini et al., 2014), the uncontrolled presence of pesticide residues and other GMOs makes the study inconclusive. Any animal parameters measured after eating GM canola cannot be compared with controls eating a diet containing other GMOs with the same characteristic, and are not taken into account. By the standards of the editor of Food and Chemical Toxicology (Hayes, 2014), this study (Delaney et al., 2014) should be retracted.
2014
Conflicts of interests, confidentiality and censorship in health risk assessment: the example of an herbicide and a GMO. Gilles-Eric Séralini, Robin Mesnage, Nicolas Defarge, Joël Spiroux de Vendômois. Editorial Environ Sci Eur. 2014. doi: 10.1186/s12302-014-0013-6.
" Conflicts of interest, confidentiality and censorship in health risk assessment: the example of a herbicide and a GMO ".
We studied the long-term toxicity of Roundup-tolerant GM maize (NK603) and a whole formulation of Roundup pesticide at environmentally relevant levels from 0.1 ppb. This has caused turmoil in the scientific editorial world, highlighting conflicts of interest...
Our study was first published in Food and Chemical Toxicology (FCT) on September 19, 2012. The first wave of criticism arrived within a week, mainly from plant biologists with no toxicology experience. We responded to all these criticisms. The debate then encompassed scientific arguments, and a wave of ad hominem and potentially defamatory comments appeared in various journals by authors with serious but undisclosed conflicts of interest. At the same time, FCT recruited a former Monsanto employee as its new Associate Editor for Biotechnology after he had sent a letter to FCT complaining about our study. Not least because of this, FCT requested a post-hoc analysis of our raw data. On November 19, 2013, the editor requested the withdrawal of our study while acknowledging that the data were not incorrect and that there was no fault, fraud or intentional misinterpretation in our raw data set - an unusual, if not unprecedented, action in scientific publishing. The publisher argued that no conclusions could be drawn because we studied 10 rats per group for 2 years, because they were Sprague Dawley rats and because the data were inconclusive on cancer. This, however, was known at the time our study was submitted. However, our study was never considered a carcinogenicity study. We never used the word "cancer" in our paper. The present opinion is a summary of the debate that led to this retraction, as it is a historical example of conflicts of interest in scientific assessments of products marketed worldwide. We also show that the decision to retract cannot be rationalized on any discernible scientific or ethical basis. Censorship of health risk research undermines the value and credibility of science, so we are republishing our article.
2014
Conclusiveness of toxicity data and double standards. Séralini, G.-E., Mesnage, R., Defarge, N., Spiroux, J. (2014) Food and Chem. Tox. 69:357-359.
" Conclusive toxicity data and double standards ".
We comment on the arguments of Mr Hayes, director of Food and Chemical Toxicology (Hayes2014) who took the decision to retract our study (Seralini et al., 2012). This publication highlights a double standard and inequity in choices to retract scientific publications.
Conclusiveness of toxicity data and double standards
Food and Chemical Toxicology, Volume 69, July 2014, Pages 357-359
G.E. Séralini, R. Mesnage, N. Defarge, J. Spiroux de Vendômois
2015
Using the livers and kidneys of rats from our 2012 study, a transcriptomic study highlighted and confirmed our findings...
Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Robin Mesnage, Matthew Arno, Manuela Costanzo, Manuela Malatesta, Gilles-Eric Séralini, Michael N Antoniou. Environ Health. 2015. doi: 10.1186/s12940-015-0056-1.
" Transcriptome profile analysis reflects rat liver and kidney damage following chronic exposure to Roundup at very low doses ".
2018
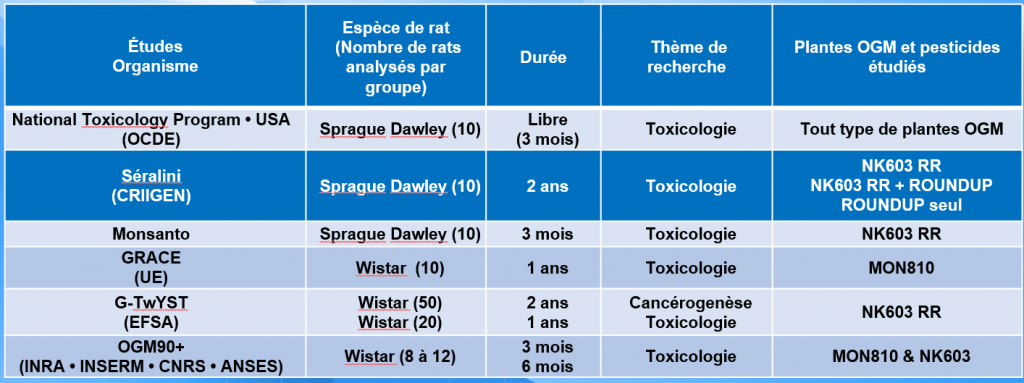
Following the republication of our study, the "pro-GMOs" launched a battle to discredit it. France and Europe decided to carry out three studies: GRACE, G-TwYST and OGM90+, in order to clarify the situation.
At the end of June 2018, following the release of the first results of the GRACE, G-TwYST and GMO90+ studies, some of our detractors once again fiercely and vehemently discredited us.
They forgot to : " Compare like with like"!
In fact, the GMO90+ study and the European studies (GRACE and G-TwYST) are not comparable with "Séralini 2012":
- The GRACE study looked at another GMO that produces its own insecticide (Bt) and is not Roundup-tolerant.
- The G-TwYST study aimed to assess carcinogenicity, a more specific question which was indeed carried out over 2 years, but the general toxicology component (with regular urine and blood tests) did not exceed one year, instead of 2 years in the Séralini study. It should be pointed out that experimental protocols focusing on carcinogenesis or general toxicology are quite different. Furthermore, the Séralini 2012 study never mentioned cancer. The word cancer does not appear in this publication; the tumors were mammary fibroadenomas, which is quite different.
- The GMO90+ study (Salles et al. 2018) is very different from the Séralini study and cannot be scientifically compared with it.
The table below shows and proves that these studies are in no way comparable with our own.
All the more so as none of these studies tested the impact of Roundup in its overall formulation in drinking water (at the equivalent dose to tap water) for two years...
In conclusion, it's sad and dangerous that scientists and science journalists should have such arguments. We're in favor of adversarial debate, but the rules of the game must be respected. How can we maintain that 3-month studies, even if they show no impact, can be used to declare the harmlessness of a GMO eaten for a lifetime (30 months)? Furthermore, in their conclusion (Salles et al. 2018), consider that the fact of having tested two GMOs for 3, or even 6 months, makes it possible to assert that all GMOs are without effects on health. We can't both criticize the Nouvel Obs in 2012 for its general headline ("GMOs are poisons") and adopt the same approach. They question the need for 3-month toxicological studies. This is perhaps the only point of agreement we have : 3-month studies are acute toxicity studies that do not allow us to assess possible chronic effects . And it's likely that the potential health effects of a GMO won't result in sudden death and screams of pain!
All GMOs should be tested for life in rats. And once again, even if studies conclude that NK603 corn has no health effects, what scientific principle should be applied to all GMOs? Is there no difference between NK603 corn, which is only tolerant to Roundup, and Smarstax corn, which contains 6 pesticidal traits: tolerance to two herbicides and the production of 6 insecticides?
Contradiction is the lifeblood of democracy and has always helped science to advance. But only if experience and the rigor of the scientific approach prevail over ideological considerations. These studies cost a total of 15 million euros. This would have been more than enough to really repeat the Séralini study (and even several times) to know definitively whether the conclusions of the 2012 study were confirmed or not! Once again, the lobbies are trying to divert the debate from the serious public health risks posed by products that regularly contaminate our ecosystems and our food, and that are found in the blood and urine of the world's population.
Conclusion of this "affair
Our study tested a GMO for 2 years and also its associated Roundup in its complete formulation, not just glyphosate alone. A world first...
It was declared inconclusive, because the statistical differences between control and treated rats were too small to be compatible with a biologically acceptable statistical difference...
This argument is unsupportable in hindsight, as we have shown (see "Regulatory Toxicology" Mesnage R et al. 2015.) that control rats are fed a pathogenic diet, which has the effect of reducing the statistical differences between controls and treated rats.
We also showed that transcriptomic analyses highlighted liver and kidney abnormalities, concerning rats testing Roundup for 2 years. See above (Mesnage et al. 2015).
Clearly, this republished study, the only one of its kind in the world, should have convinced the world's health safety agencies to modify their toxicological tests and therefore to test all pesticides in their global formulations and GMOs for two years.
This is still not done at the expense of global health...
